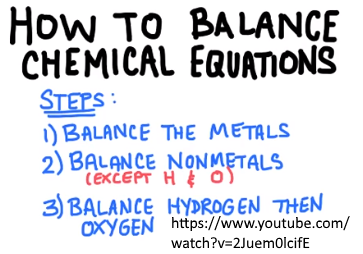
Instructions: After the mentioned metal reacted with air, write down the chemical name and formula of the product and state its observable change.
Mark the third blank yourself.
e.g.: Question: Calcium --> Answer: reacts, Calcium Oxide, 2Ca(s)+O2(g)->2CaO(s), Calcium burns with ..., ... forms.